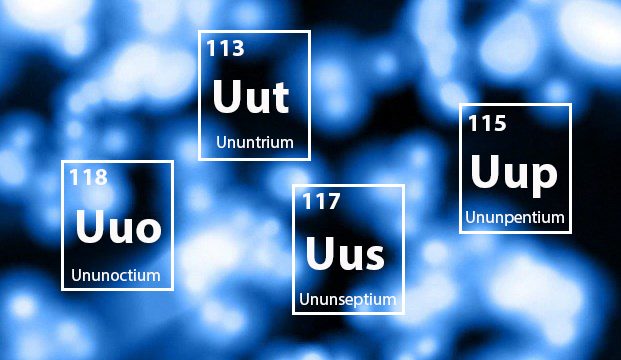
Scientists announced the addition of four new elements to The Periodic Table of Elements, which will sit in spots 113, 115, 117, and 118. Professor of Chemistry Gregory Ferrence gives some insight into the birth of these new, and as yet unnamed, elements, and compares them to astrophysicists who reach for the stars.
Ferrence:
It really is a big deal, scientifically, to add four new elements to the Periodic Table.
Let me give you a visual to help explain this. Imagine that the middle part of an atom—the nucleus—is a suitcase. We define an element by how many protons you have in that suitcase. If you have six protons, you have carbon; eight protons is oxygen, etc.
Now, for a long time, the periodic table rarely added elements. After World War II and the Manhattan Project, however, scientists realized they could use nuclear processes to create elements that never before existed. Essentially, we found a way to stuff more protons into the suitcase.
Of course, the more protons you shove into a suitcase, the more full it gets. If you are like me, you still think you can get that one extra shirt or sock in there. But what happens when you stuff the suitcase too full? It bursts.
Scientists at the large colliders, like Lawrence Livermore National Laboratory, the Joint Institute for Nuclear Research in Russia, and RIKEN in Japan, are shooting atoms at one another in the hopes that they can get that extra “sock” of a proton in the suitcase. And they have. The new element in slot 113 has 113 protons. The new element in 118 has 118 protons. But you can’t expect a suitcase that full to last.
A moderately unstable element like uranium, with 92 protons, typically decays over billions of years. But these super-stuffed suitcases tend to fall apart in seconds or less. Element 118 lasts only for a fraction of a millisecond.
It took scientists a long time to find the new elements. Let’s put it this way, in a dime, you will find 22 billion trillion atoms. In these large collider experiments, after three to four months of experimentation, they may find only three to four 118 atoms. Now there could be thousands, but the suitcases may be exploding before they can be detected.
It’s also quite incredible that four elements are added to the table at once, but not surprising. In order to prove that a new element exists, scientists usually watch how an element decays. A nucleus falls apart in certain, predictable ways. So scientists look for the decay chain—the path that will lead them back to the new element.